Potassium ion channels on cell membranes exhibit a unique property known as inward rectification. This means that they are more effective at transporting ions into the cell than out of it, due to the blockade of the channel pore by intracellular cations such as Mg2+ and polyamines. Drawing inspiration from this phenomenon, Prof. Genxi Li's group at the School of Life Sciences, Nanjing University, collaborated with Prof. Farid A. Harraz from Najran University in Saudi Arabia and Assoc. Prof. Tao Gao from Nanjing Normal University, proposed a biomimetic biosensing platform for detecting tumor biomarkers. The group accomplished this by designing peptides with various isoelectric points and introducing them into artificial nanochannels. By modifying the peptides, the researchers were able to give them the ability to modulate the inward rectification current, which allowed the platform to be successfully used to detect specific types of enzymes. This groundbreaking approach could have significant implications for the diagnosis of cancer.
Peptides possess electrical properties that are reflective of their biological functions, owing to their characteristic isoelectric points that vary depending on the pH of the biological system. Additionally, due to the characteristics of easy modification, diverse assembly and bio-friendliness of peptides, the assembly of peptides in the nano-confined space can be regulated by thermodynamic and kinetic factors to achieve a variety of biological functions. As shown in Figure 1, functional peptides were modified and assembled into artificial nanochannels in this study. At physiological pH, peptides with different isoelectric points resulted in opposite charges on the surface of the nanochannel. Nanochannels modified with positively charged peptides exhibited anion selectivity and the rectification effect of anion channels, while nanochannels modified with negatively charged peptides exhibited a reversal of the ion rectification direction, as demonstrated in the current-voltage (I-V) curve. By incorporating peptides containing specific enzyme cleavage sites in nanochannels, the alteration of the isoelectric point of peptides under the action of specific enzymes enabled the detection of the enzymes via changes in the electrical properties of the nanochannels. The research groups demonstrated that this approach could be used to reliably detect tumor biomarkers and hold promise for future development in biosensing applications.
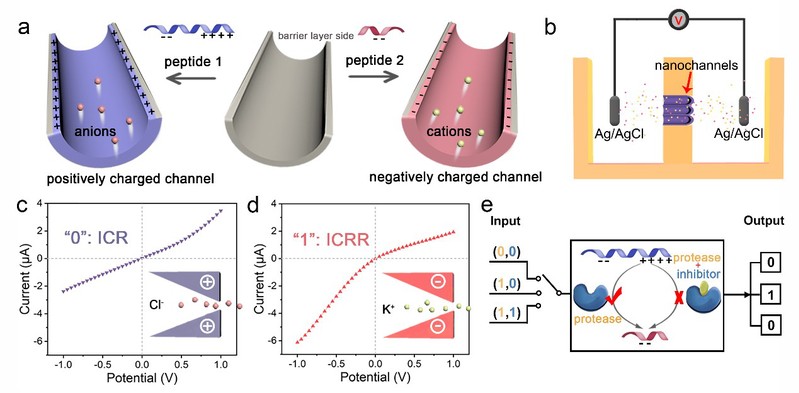
Figure 1. Principle of electrochemical biosensing based on ion current rectification reversal of the peptide-functionalized nanochannels.
Prof. Genxi Li and his research group have long been engaged in research on biomolecular engineering and applications in disease diagnosis. Therefore, using the detection of the tumor marker matrix metalloproteinase-2 (MMP-2) as an example, the group has designed a substrate peptide containing the cleavage site of MMP-2 on the nanochannels (Figure 2). The substrate peptide has a higher isoelectric point than the physiological pH, resulting in a positive charge on the surface of the nanochannel. However, under the catalysis of MMP-2, the substrate peptide is cleaved between the Gly and Val in the peptide, resulting in the loss of positively charged amino acids and a change in the isoelectric point of the remaining peptide on the channel surface. Therefore, under physiological conditions, peptide cleavage can change the polarity of the surface charge of the nanochannels from positive to negative, which can be visually reflected in the I-V curve.
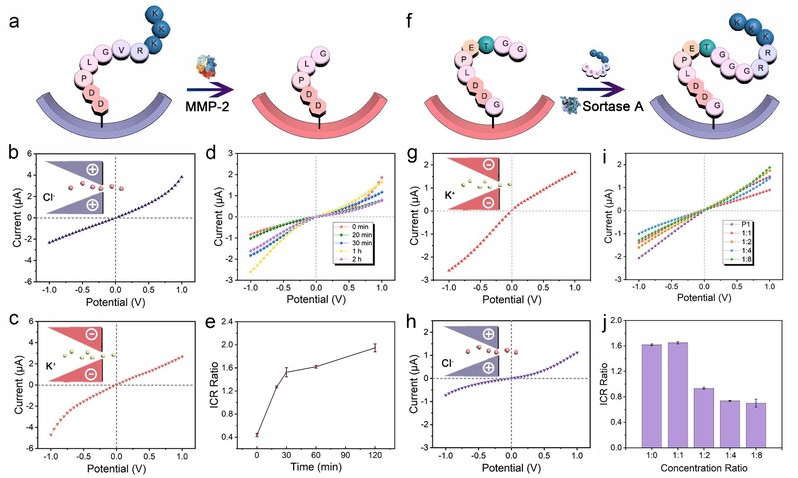
Figure 2. Detection of tumor biomarkers based on peptide-functionalized nanochannels.
The latest research has revealed that the sensing platform has the potential to not only detect proteases effectively but also screen enzyme inhibitors. Moreover, the platform relies on ion rectification reversal generated by the isoelectric point change of peptides as the output signal of the biosensor, which makes it more accurate and intuitive. In addition, due to the rich physicochemical and biological properties of peptides, peptide assembly can be designed to present more novel biological sensing interfaces, which can be applied to a wider range of analysis and detection of disease biomarkers.
The above-mentioned achievement, entitled Peptide Assembled in Nano-Confined Space as Molecular Rectifier for the Availability of Ionic Current Modulation, was published in Nano Letters (https://pubs.acs.org/doi/abs/10.1021/acs.nanolett.1c04154). Prof. Genxi Li, Assoc. Prof. Tao Gao from Nanjing Normal University, and Prof. Farid A. Harraz from Najran University in Saudi Arabia are the corresponding authors. The first author of the paper is Liu Shi, a doctoral student from Nanjing University. This work was supported by the Saudi Arabian Ministry of Education Research and Innovation Fund for International Cooperation Projects, as well as grants from the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, and the Natural Science Foundation of Jiangsu Province.
