Type II polyketide synthases (PKSs) are independent single-function enzymes that form multi-enzyme complexes capable of assembling various biologically active aromatic natural products, such as clinically important compounds like actinomycin, tetracycline, and mitomycin. In typical type II PKS systems, polyketide synthase ketosynthase (KS) and chain length factor (CLF) form a heterodimer (KS-CLF) that catalyzes multiple rounds of decarboxylative Claisen condensation to generate a polyketide acyl thioester intermediate that is attached to an acyl carrier protein (ACP). The full-length polyketide intermediate is then folded into an aromatic compound with the aid of ketoreductases (KRs), cyclases (CYCs), and aromatases. In recent years, a class of highly reduced type II PKSs (HR Type II PKSs) has emerged, which have been shown to be involved in the biosynthesis of polyene compounds. For example, biochemical studies of the in vitro synthesis of the polyene compound ishgamide have shown that an HR Type II PKS is responsible for its biosynthesis. The core enzymes of the Iga II PKS system have much in common with typical type II PKS systems, but have additional KRs and dehydratases (DHs). KRs and DHs catalyze the repeated β-keto reduction and dehydration of the substrate on ACP to form double bonds, ultimately forming polyene compounds after multiple extension cycles.

In addition, in recent years, a small number of natural products containing cinnamoyl groups (CCNPs) have been reported, such as skyllamycin, WS9326A, haoxinnamide, pepticinnamin, atratumycin, youssoufene, etc., all of which have antibacterial, cytotoxic, and anti-tuberculosis activities. The biosynthesis of cinnamoyl groups is believed to be catalyzed by a special highly-reducing (HR) type II polyketide synthase (PKS) (Scheme 1). However, the biosynthetic pathway, particularly the cyclization step for the formation of the phenyl ring, remains unclear.


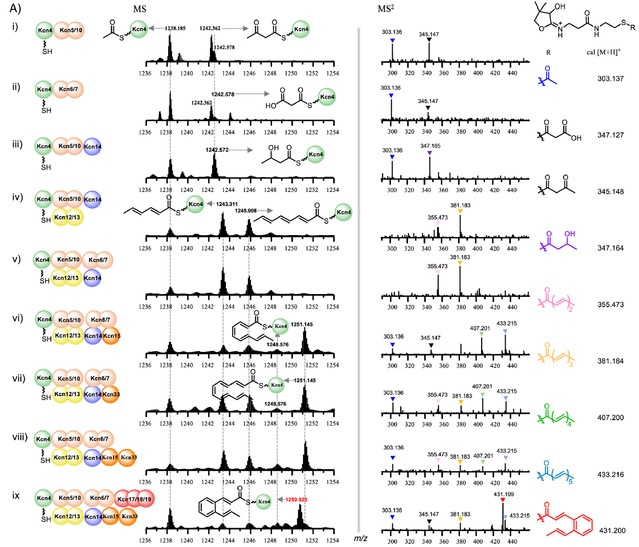
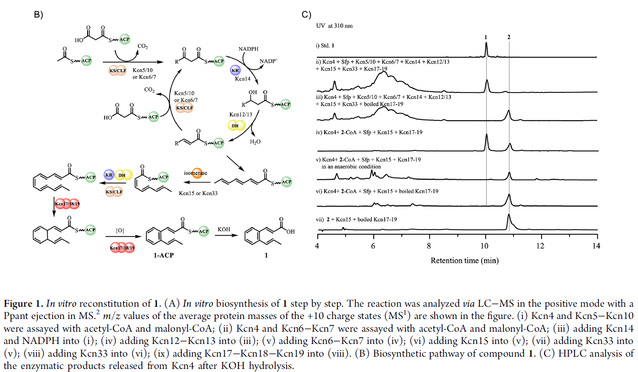
In previous studies, we discovered a class of glycopeptide compounds containing cinnamoyl groups called kitacinnamycins through gene mining (Chem. Sci., 2019, 10, 4839-4846.). Since the biosynthetic pathway of the special cinnamoyl groups in these CCNPs has not been fully elucidated, we used kitacinnamycin as a model compound to decipher its biosynthetic pathway. In this study, we expressed and obtained the proteins related to HR Type II PKS on the kcn gene cluster, and successfully reconstituted the synthesis of the cinnamoyl group in kitacinnamycin in vitro. In summary, using acetyl-CoA and propionyl-CoA as substrates, ACP, KS/CLF, KR, and DH synthesized a C8-trienyl intermediate through three extensions. The third double bond was isomerized by an isomerase, followed by two more extensions to form the C12-pentadienyl substrate. Finally, the three-protein complex Kcn17-Kcn18-Kcn19 catalyzed the 6π-electrocyclization and dehydrogenation of the C12-pentadienyl substrate to form the benzene ring (Figure 1).
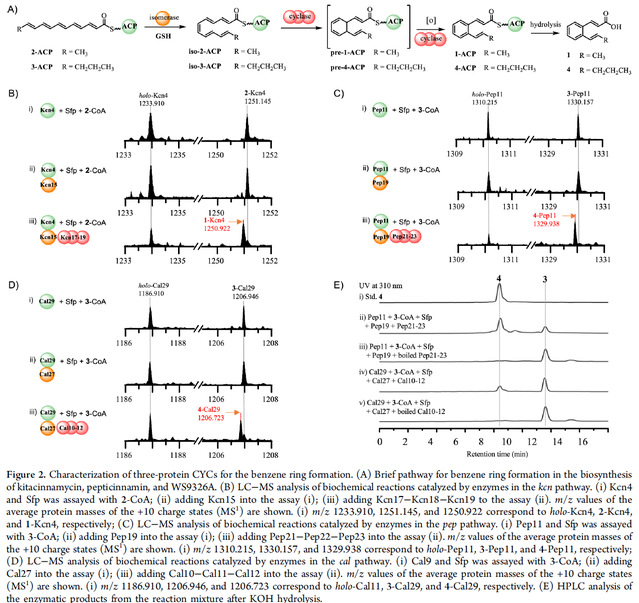
Encouraged by the catalytic function of the Kcn17-Kcn18-Kcn19 complex, we considered whether other homologous proteins could also catalyze the formation of the benzene ring. Therefore, we expressed the relevant proteins from the biosynthetic gene clusters of compounds pepticinnamin and WS9326A and chemically synthesized possible polyene substrates 2 and 3. By testing the functions of the corresponding isomerases and three-protein complexes in vitro using 2 and 3 connected to the corresponding ACPs as substrates, we were able to produce the corresponding cyclization products. Thus, our results show that three protein complexes, Pep21-Pep22-Pep23 and Cal10-Cal11-Cal12, are responsible for the formation of the cinnamoyl part of the benzene ring in pepticinnamin and WS9326A biosynthesis, respectively.
Unlike the cinnamoyl portion that is bound to amino acid residues through amide bonds, youssoufene is a unique free cinnamic acid derivative. Previous literature reported that the polyene intermediate in the biosynthesis of youssoufene was catalyzed by an HR Type II PKS, but the enzyme responsible for the formation of the benzene ring remained unknown. We found that there was no homologous protein of Kcn17-Kcn18-Kcn19 in its biosynthetic gene cluster, but compared with the previously reported ysf gene cluster, our yss gene cluster had an additional small protein YssX containing only 59 amino acids. Knocking out yssX resulted in the absence of the final product, indicating that YssX is involved in the biosynthesis of youssoufene. To verify the function of YssX, we reconstructed the biosynthetic process of youssoufene in vitro, and similar to the function of Kcn17-Kcn18-Kcn19, YssX catalyzed the formation of the benzene ring. Thus, we have elucidated the complete biosynthetic pathway of youssoufene.

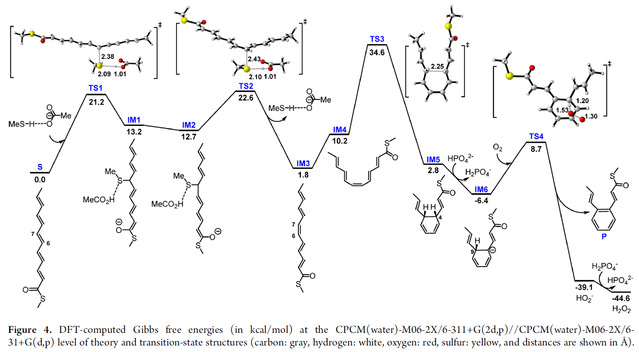
To better understand the reaction mechanism of phenyl ring formation, we performed density functional theory (DFT) calculations on a series of reactions starting from polyene S (mimicking 2-ACP), which further supports the catalytic role of the three-protein complex Kcn17-19 or protein YssX in biosynthesis.
In this study, we successfully reconstituted the biosynthesis of cinnamoyl moiety in vitro and extended our findings to other CCNPs, revealing the long-standing mystery of benzene ring formation in cinnamoyl biosynthesis. Although benzene rings are ubiquitous in natural products, their biosynthesis is not easy, and nature employs two main methods to synthesize benzene rings: (1) the shikimate pathway, a common pathway for the generation of aromatic amino/hydroxy acids, which are the major sources of most biologically produced benzene derivatives; (2) the other pathway is through PKSs-catalyzed cyclization of active polyketide backbones.
This study first demonstrated that two cyclases from completely different evolutionary systems can synthesize benzene rings through the biosynthesis of polyene precursors. The cyclization catalyzed by the trimeric complex represents a relatively common strategy for forming benzene rings in CCNPs, as at least six identified and 207 unidentified biosynthetic gene clusters contain this trimeric manipulation. YssX is a standalone enzyme that is responsible for benzene ring formation only in the biosynthesis of youssoufene. Our results expand our understanding of aromatic ring biosynthesis and lay the foundation for further genome mining and combinatorial biosynthesis of CCNPs.
Dr. Jing Shi, an associate researcher at the School of Life Sciences, Nanjing University, is the first author of the paper, and Professors Hui Ming Ge and Ren Xiang Tan from the School of Life Sciences, Nanjing University, and Professor Yong Liang from the School of Chemistry and Chemical Engineering, Nanjing University, are the corresponding authors of the paper. This research was supported by the National Key R&D Program, the National Natural Science Foundation of China (Outstanding Youth Fund and Youth Fund), and the Fundamental Research Funds for the Central Universities.
Link to the original article: https://pubs.acs.org/doi/pdf/10.1021/jacs.2c02855
Title of the article: In Vitro Reconstitution of Cinnamoyl Moiety Reveals Two Distinct Cyclases for Benzene Ring Formation
