
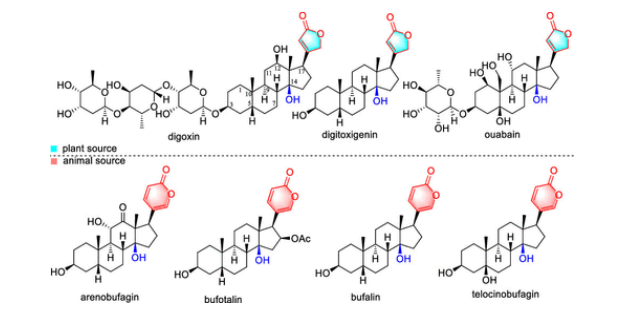
Strophanthidin is a special family of metabolic products widely distributed in nature, which contain a characteristic C14β-OH in the steroid skeleton and are often linked to a five- or six-membered unsaturated lactone ring at the C17 position (representing two types of strophanthidin structures), and may be modified by a sugar unit at the C3 position. Strophanthidin molecules produced by plants often contain a γ-butyrolactone ring at the C17 position, while those produced by amphibians such as toads contain a δ-valerolactone unit at the C17 position (Figure 1). For many years, these molecules have mainly been used as cardiotonics. With the development of their anticancer, anti-inflammatory, and antiviral activities, the rich and good biological activities of strophanthidin molecules are currently active in many clinical studies. However, the current industrial production of these drugs still relies on in-situ purification from animals and plants, which severely limits the application of strophanthidin molecules. In addition, small differences in strophanthidin structure greatly affect their physiological activity. Therefore, more and more research is dedicated to exploring and deciphering the biological and chemical synthesis processes of strophanthidin complex structures to provide new ideas for their more efficient industrial production.
On July 28, 2022, the latest research results of the Hui Ming Ge team on strophanthidin steroids were published online in the ACS Catalysis journal. By biocatalyzing the C14 position of the steroid small molecule androsta-4,9(11)-dien-3,17-dione, and then using chemical methods to construct an efficient synthetic pathway, the method was successfully applied to the synthesis of two types of natural strophanthidin products - bufotalin, bufogenin B, digitoxigenin (Figure 2).

In previous studies, the biosynthesis of strophanthidin molecules mainly focused on the formation of pregnenolone and other steroid intermediates from cholesterol precursors, and there were few reports on the assembly of the characteristic C14 hydroxyl group and the unsaturated lactone ring at the C17 position. Therefore, researchers focused on the formation of the characteristic C14β-OH of this type of molecule and explored the P450 enzymes that might catalyze hydroxylation. First, the plant Calotropis gigantea, which can produce strophanthidin molecules, was selected as the research object, and the transcription levels of its different tissues were analyzed. Finally, through functional verification of 11 candidate P450 genes and feeding with pregnenolone and androsta-4,9(11)-dien-3,17-dione, which are the strophanthidin biosynthetic intermediates and structural analogs, it was determined that CYP11411 can catalyze the production of α-OH at the C14 position of the steroid, which is opposite to the target configuration. The researchers also studied the host animal of another type of strophanthidin, the toad, and found that CYP44476 could catalyze the hydroxylation of the steroid substrate at the C14 position, as well as at the C6, C9, and C15 positions. Based on the study of hydroxylases in animal and plant hosts, it is speculated that nature may preferentially directly produce α-OH by seizing α-H, and then complete the configuration inversion through dehydration and hydration pathways.
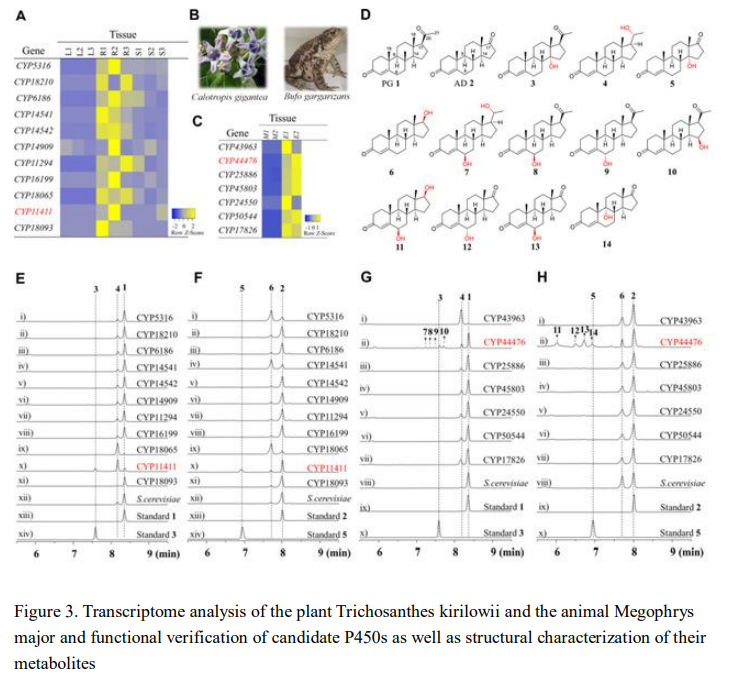
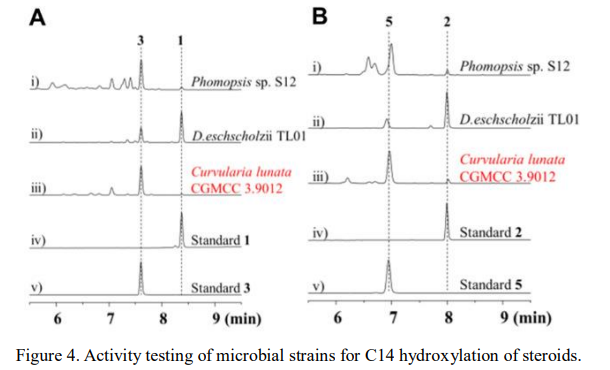
The team first discovered the C14 hydroxylase responsible for producing cardiac glycosides in the host, which can directly achieve site-specific oxidation of inert steroid skeletons, a step that is difficult to accomplish through chemical reactions. To synthesize cardiac glycoside molecules, the researchers plan to complete the large-scale synthesis of C14 hydroxylated steroids through biocatalysis and then assemble the oxygen-containing heterocycle at the C17 position through chemical means. Therefore, it is necessary to scale up the production of C14 hydroxylated steroids. Metabolic profiling analysis of the hydroxylase through heterologous expression revealed low conversion efficiency, so the researchers transferred the target to a green and efficient microbe for biotransformation. After screening microbial strains and hydroxylation activity testing, they ultimately discovered that the fungus Curvularia lunata could efficiently catalyze the production of the target product (Figure 4), and after further optimization, the separation yield could reach 70%, obtaining 5g of C14α-OH-androsta-4,16-diene-3,17-dione.
Next, the researchers proceeded to assemble the five- or six-membered unsaturated lactone ring at the C17 position using chemical synthesis. First, the A/B cis ring system was constructed by the ∆4,5 hydrogenation reduction of substrate C14α-OH-androst-5-en-17-one, followed by the selective reduction of the C3 ketone and hydroxyl groups to obtain compound 17 with a silicon ether protection. To achieve the critical C14 hydroxyl configuration flip, the α-OH was eliminated via dehydration and then the β-OH product was generated through Mukaiyama hydration. The isomer ratio of the product in the alkene hydration process is closely related to the reaction solvent. Starting from the alkene iodine intermediate 21, the five- or six-membered lactone ring can be respectively coupled (as shown in Figure 5).

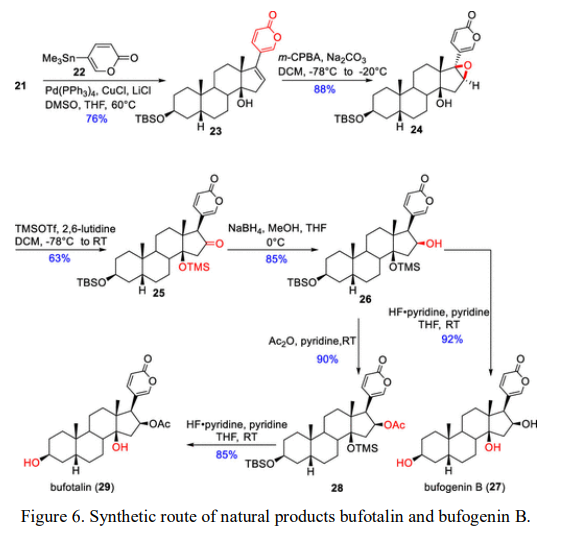
To complete the synthesis of cardiac glycosides, the basic skeleton of cardiac glycosides was constructed through the Stille coupling reaction of compound 21 with tin reagents. The ∆16,17 double bond was oxidized to form a three-membered oxygen ring, followed by 1,2-hydride shift and ring-opening rearrangement via TMSOTf and 2,6-lutidine-catalyzed formation of C14 silane ether. Finally, natural products bufotalin and bufogenin B were obtained through C16 carbonyl reduction, acetylation, and deprotection of protecting groups (Figure 6).
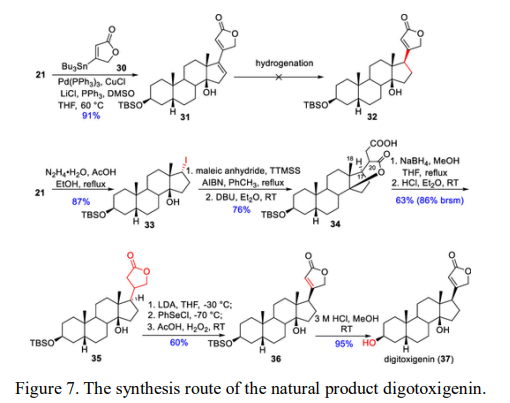
The alkyl iodide formed from compound 21 is more prone to free radical reactions. Compound 33 undergoes a SN2-like radical coupling reaction with maleic anhydride in the presence of the radical donor TTMSS and the initiator AIBN. Then, under basic conditions, the resulting C14 hydroxyl group participates in forming an internal lactone ring. Selective reduction and lactonization with NaBH4-MeOH (via an opening and reclosing process) forms 35, and the phenyl selenyl chloride derivative is further oxidized to obtain the unsaturated lactone ring product, which is finally obtained as digotoxigenin after acid hydrolysis (Figure 7).
In summary, the research team identified for the first time two P450 enzymes, CYP11411 and CYP44476, capable of catalyzing C14 hydroxylation in cardenolides from natural hosts, laying the foundation for elucidating their biosynthesis pathways. Meanwhile, using microbial efficient transformation, they synthesized the steroid C14α-OH-androstenedione and used it as a substrate to assemble the unsaturated lactone ring units to complete the synthesis of two types of cardenolide natural products. This approach of using both biocatalysis and synthetic chemistry can be applied to create more bioactive cardenolide molecules and their structural analogues.
Yang Zhao, a Ph.D. student from the School of Life Sciences at Nanjing University, is the first author of the paper, and Professors Hui Ming Ge and Ren Xiang Tan from the School of Life Sciences at Nanjing University and Professor Shou Yun Yu from the School of Chemistry and Chemical Engineering are the corresponding authors of the paper. This research was supported by the National Key R&D Program, the National Natural Science Foundation of China Outstanding Youth Fund, the Youth Fund, and the Fundamental Research Funds for the Central Universities.
Original article link: https://doi.org/10.1021/acscatal.2c02185
Article title: Biocatalytic C14-Hydroxylation on Androstenedione Enabled Modular Synthesis of Cardiotonic Steroids
