Benzene rings are widely present in organisms, and their aromatic conjugated structure can promote the interaction between drugs and key residues of targets, thus exerting their pharmacological effects. However, there are usually two ways to synthesize this aromatic group in nature: (1) In primary metabolism, the shikimate pathway is a common route for the synthesis of aromatic amino and hydroxy acids, which are the main sources for the biosynthesis of most phenolic derivatives. (2) In secondary metabolism, type II polyketide synthase catalyzes the aldol condensation and aromatization of polyketide skeleton to form benzene rings. Whether there are other forms of benzene ring synthesis pathways in nature is still poorly understood. Earlier, the research group led by Go Huiming discovered a new mechanism that can catalyze the formation of benzene rings through 6π-electrocyclization and oxidation of polyene-containing compounds during the biosynthesis study of natural products containing cinnamoyl groups(J. Am. Chem. Soc. 2022, 144, 7939–7948).Recently, focusing on this scientific problem, they further discovered a type of cytochrome P450 enzyme that can efficiently and simply catalyze the formation of trialkyl-substituted benzene rings.
In the earlier research, it was found that Lorneic acid is a type of polyketide antibiotic containing a 1,2,4-trialkyl-substituted benzene ring with broad biological activity (Figure 1). The characteristic of such molecules is that the benzene ring is located in the middle of the entire skeleton, and its formation cannot be directly introduced from external sources, but is more likely to be formed through a novel mechanism in the later stages.

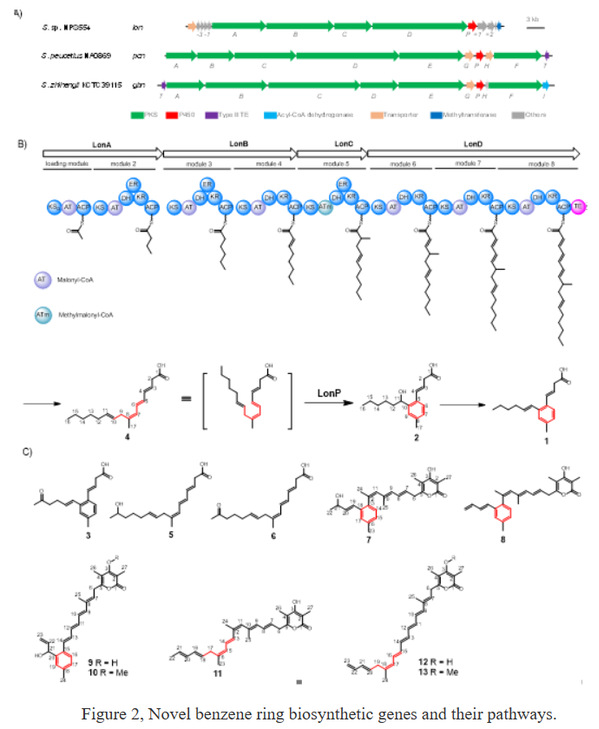
The team's research found that the P450 enzyme LonP can catalyze the aromatic ring formation of linear polyenes 4-oxidation to produce lorneic acid (2) containing benzene rings (Figure 2). Based on this, the researchers speculated that the benzene rings in such molecules in nature are formed by P450 enzyme catalysis. To verify this hypothesis, the researchers used LonP as a probe for analysis and identified 12 homologous gene clusters containing conserved P450 enzymes, suggesting that these gene clusters are likely responsible for synthesizing structural analogues of lorneic acid. After screening and optimization of the culture medium, the researchers identified four new polyene antibiotics containing trisubstituted benzene rings. Gene knockout and in vitro biochemical experiments confirmed that cytochrome P450 enzymes catalyze the formation of benzene rings. Thus, the researchers have discovered a novel mechanism in which benzene rings are formed by cytochrome P450 enzymes in nature.
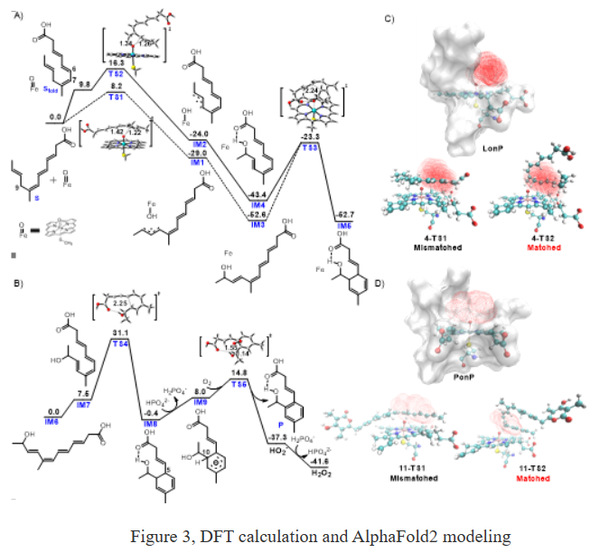
To elucidate the catalytic mechanism of these cytochrome P450 enzymes, researchers first used quantum calculations to find that when the substrate enters the enzyme's active pocket with a high-energy folded conformation and then undergoes hydroxylation and 6π-electrocyclization reactions, it can significantly reduce the energy barrier of the electrocyclization and thus accelerate the reaction. AlphaFold2 modeling and molecular dynamics simulations also suggest that compared to the transition state TS1, the restricted active pocket of the P450 enzyme is more accommodating to the transition state TS2. Therefore, this type of P450 enzyme mainly uses the advantage of specific binding of substrate conformation to reduce the energy barrier of the electrocyclization, and efficiently constructs benzene rings through hydroxylation, electrocyclization, and dehydrogenation cascade reactions (Figure 3).
This work provides a clear elucidation of the biosynthetic mechanism of lorneic acid and other natural products of the same class, revealing a novel strategy for the formation of benzene rings in nature, and greatly enriching the functional diversity of the P450 superfamily. The related research was published in Angewandte Chemie International Edition(Angew. Chem. Int. Ed. 2023, 62, e202214026). Yu Meng Yang, Er Juan Zhao, and Wan Qing Wei, doctoral students from the School of Life Sciences and the School of Chemistry and Chemical Engineering of Nanjing University, are the first authors of the article, and Professor Hui Ming Ge and Professor Rui Hua Jiao from the School of Life Sciences and Professor Yong Liang from the School of Chemistry and Chemical Engineering of Nanjing University are the corresponding authors. The research was supported by the National Key Research and Development Program, the National Natural Science Foundation of China, and the Fundamental Research Funds for the Central Universities, among other projects.
Original article link:https://doi.org/10.1002/anie.202214026
