Innate immunity is the first major attack of the host against invading pathogens. Viral genomes are important pathogen-associated molecular patterns and a series of nucleic acid sensors exist in host cytoplasm to recognize viral nucleic acids and initiate immune response pathways. However, some DNA viruses, such as herpesvirus (HSV) and human adenovirus (HAdV), can evade the recognition by DNA sensor in host cytoplasm by injecting viral DNA directly into the nucleus. However, the current research on DNA sensors in the nucleus is still not well studied.
Recently, heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNP A2/B1) has been identified as a nuclear DNA sensor that can recognize viral DNA in the nucleus and form homologous dimers. The hnRNP A2/B1 homodimer is the prerequisite for its translocation to the cytoplasm to activate interferon signaling pathway. However, the molecular mechanism by which hnRNP A2/B1 recognizes DNA and forms homodimers remains unclear.
Professor Xiaoyun Ji from the State Key Laboratory of Pharmaceutical Biotechnology and School of Life Sciences, Nanjing University has been studying the molecular mechanism of viral infection and host innate immune response. Recently, the team obtained the crystal structure of hnRNP A2/B1 in complex with single-stranded DNA (ssDNA) by X-ray diffraction and further confirmed that the U-shape conformation of DNA is critical for the DNA-induced hnRNP A2/B1 homodimerization through biochemical experiments, depict a potential functional state of hnRNP A2/B1 in antiviral immunity and other cellular processes. (Figure 1).
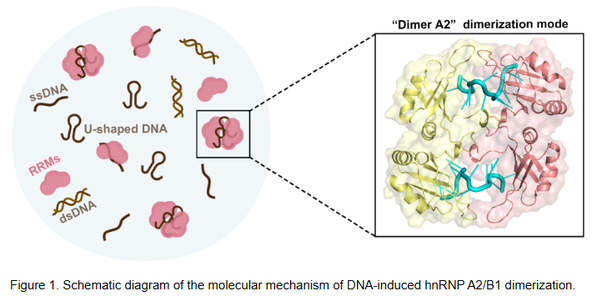
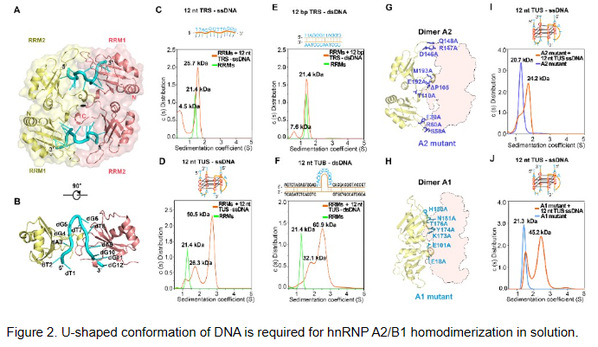
Through the high-resolution crystal structure of the nucleic acid binding structural domains (RRMs) of hnRNP A2/B1 in complex with 12 nt ssDNA, the team observed for the first time that a unique U-shaped DNA conformation mediates the formation of a novel dimerization pattern of hnRNP A2/B1 (Figure 2A-B). Biochemical experiments revealed that hnRNP A2/B1 was able to bind G-quadruplex ssDNA mimicking a U-shaped DNA conformation and form the protein homodimers in solution. However, only one molecule of protein bound to one molecule of DNA in solution while binding to linear ssDNA (Figure 2C-D). In addition, the researchers found that only double-stranded DNA (dsDNA) containing a U-shaped bulge could bind to hnRNP A2/B1 and induce protein dimer formation, while dsDNA lacking the bulge structure could not bind to hnRNP A2/B1 (Figure 2E-F). These results suggest that the U-shaped conformation of DNA plays a key role in inducing hnRNP A2/B1 dimerization.
The RRM domain is one of the most extensively studied nucleic acid binding domains. The researchers identified two potentially stable dimer assemblies of hnRNP RRMs in complex with nucleic acids (Figure 2G-H). Further mutagenesis study showed that disrupting mutations on the protein dimer interface of “Dimer A2” abrogate the dimerizing ability of RRMs induced by U-shaped ssDNA binding (Fig. 2I-J). This demonstrates that an hnRNP A2/B1 homodimer induced by U-shaped ssDNA binding in solution exists as a “Dimer A2” protein, as they observed in the crystal structure of hnRNPA2/B1 RRMs in complex with 12 nt TRS ssDNA.
The unique U-shaped DNA structure is abundant in viral genomes and human genomes such as telomeres. This study not only proposed a molecular mechanism by which hnRNP A2/B1 recognizes U-shaped conformations in ssDNA or dsDNA but also broadens the understanding of the hnRNP A2/B1 homodimer upon recognition of DNA to accomplish its antiviral and physiological functions.
The study was published in the Journal of Molecular Biology on 1 February 2023 entitled “Structural insight into hnRNP A2/B1 homodimerization and DNA recognition”. Yue Liu and Dr. Abudureyimu Abula from the School of Life Sciences, Nanjing University are the co-first authors of this article. Professor Xiaoyun Ji from Nanjing University is the corresponding author of this article. This research was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
Link to article: https://www.sciencedirect.com/science/article/pii/S0022283622005460
