Lung cancer is the leading cause of cancer-related deaths worldwide. Most lung cancers are non-small cell lung cancer (NSCLC). After diagnosis, the 5-year survival rate of NSCLC patients is less than 15%. Immune checkpoint blockers like monoclonal antibodies against programmed cell death protein 1 (PD-1) and its ligand (PD-L1) have recently demonstrated improved clinical benefits in NSCLC. However, the overall response rate to PD-1/PD-L1 blockade rarely exceeds 40%, with the main obstacle being the tumor immunosuppressive microenvironment (TIME). Tumor PD-L1 expression significantly affects the clinical efficacy of PD-1/PD-L1 immunotherapy and drug resistance. Clarifying the factors affecting PD-L1 expression in TIME is essential for improving PD-1/PD-L1-based immunotherapy.
Endocrine hormones (EHs) significantly shape TIME in cancer, however, the role of EH in TIME of NSCLC remains little understood. In endocrine-related cancers, endocrine therapy has become an important treatment option. NSCLC is non-hormonally-driven cancer, but increasing evidence demonstrated that EHs such as estrogen are important in NSCLC cell growth and therapy. Various EH receptors have further been identified on the cell membranes of NSCLC cells. As such, the importance of EH in NSCLC development and the relevant personalized treatment cannot be underestimated.
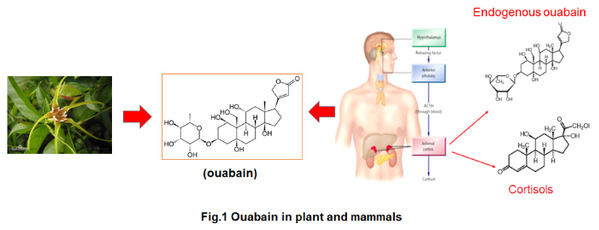
Endogenous ouabain (EO) is a newly discovered EH mainly produced by the adrenal glands. Interestinly, ouabain is also an active component produced in plant, which has has been proposed to be chemically identical to EO in mammals (Fig.1). After binding to the receptor Na, K-ATPase, low concentrations of serum EO can initiate signaling events without significantly affecting the ion transport activity Na, K-ATPase. Thus, Na, K-ATPase is also a hormone receptor. EO-mediated signaling is mainly associated with cardiovascular and renal diseases. However, it is completely unknown whether EO is involved in cancer development. Since the discovery of EO, scientists have predicted that they may have many endogenous Na, K-ATPase ligands in mammals that are chemically similar to, and share Na, K-ATPase receptors with, CGs. However, it is unclear whether these endogenous Na, K-ATPase ligands influence the clinical efficacy and safety of CGs in vivo. Without this knowledge, it is difficult to determine whether CGs may be used in cancer treatment.
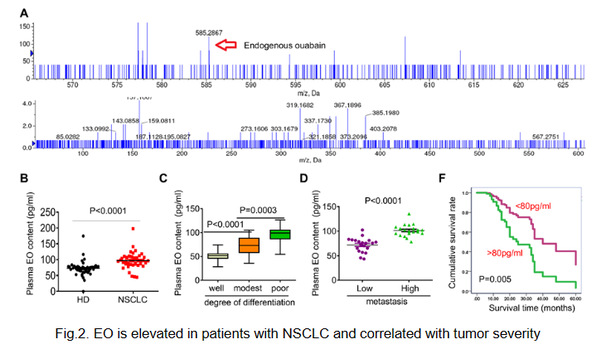
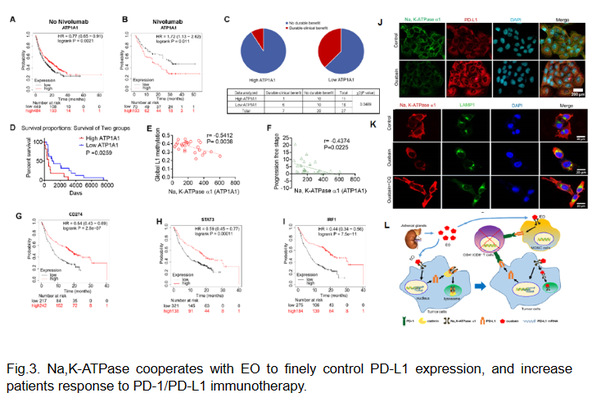
Recently, we described that serum EO was elevated in patients with NSCLC or immunologically intact tumor-bearing mice, which is well correlated with tumor metastasis, differentiation, and prognosis (Fig.2). Unlike the antitumor effects of CGs, EO was found to promote the development of NSCLC. In cooperation with Na, K-ATPase, EO plays an important role in reprogramming tumor immunity toward an immunosuppressive phenotype by finely controlling PD-L1 expression, on tumor or suppressive immune cells, at both transcriptional and post-translational levels in TIME (Fig.3). The Na, K-ATPase/EO signaling pathway therefore represents an endocrine pathway that can be exploited by lung cancer cells to facilitate immune escape. Based on this finding, EO antagonism and the combined use of CGs or cinobufacini injections with anti-PD-L1 IgG shows great promise for the immunotherapy of NSCLC.
The paper was published on February 10, 2023 in Science Advances titled with Na,K-ATPase α1 cooperates with its endogenous ligand to reprogram immune microenvironment of lung carcinoma and promotes immune escape (Sci Adv.2023 Feb 10; 9(6):eade5393.). This study expands the new biological function of EO, and provides a theoretical basis for discovering a new mechanism of tumor immune escape and improving the effect of lung adenocarcinoma immunotherapy. The study is supported by the key project of the National Natural Science Foundation of China and the key research and development plan of Jiangsu Province. The first authors are Yang Kaiyong and Li Zijian, and Professor Yin Wu is the corresponding author. Professor Yin Wu has been engaged in the research of new biological functions of Na,K-ATPase and its ligands for a long time, and the work has been published in top journals including EMBO Mol Med, Leukemia, J Am Soc Nephro. and so on. , also obtained many patent authorizations.