At present, the diagnostic methods for breast cancer are mainly based on imaging technology, tissue biopsy technology and genetic screening technology, which are mature but also have some shortcomings, so there is an urgent need to develop new diagnostic technologies. Human epidermal growth factor receptor 2 (HER2) is a transmembrane protein that is overexpressed in about 20-25% of breast cancers. A large amount of evidence shows that accurate detection of HER2 in the early stage has a significant impact on improving the survival of breast cancer patients. Recently, Prof. Genxi Li from the School of Life Sciences of Nanjing University, in cooperation with Prof. Jing Zhao from Shanghai University and Prof. Yongmei Yin from Jiangsu Provincial People's Hospital Affiliated to Nanjing Medical University, constructed a colorimetric biosensor with excellent analytical performance based on DNA framework-controlled Poly (MOFs), which can realize the analysis of HER2 at the molecular level and the cellular level, and be used for the diagnosis of HER2-positive breast cancer patients.
Metal-organic frameworks (MOFs) have received extensive attentions in biomedical fields such as biosensing, medical imaging, and drug delivery due to their extremely high specific surface area, ultra-high porosity, and tunable pore structure. Many valuable advances have also been made in design, synthesis, modification, and function of MOFs. Beyond all doubt, the successful nano-bio interface engineering of MOFs for improving stability, target capability, and biocompatibility is fundamental for biomedical applications. Up to now, there have been only a few studies on the engineering and application of MOFs nano-bio interfaces, which mainly use functional probes such as nucleic acids, peptides, and antibodies. There are still many obvious difficulties and challenges in MOFs nano-bio interface engineering: (1) a finite number of active groups on the surface of MOFs for functionalization; (2) the low recognition efficiency of nanoprobes caused by the surface adsorption of MOFs; (3) rare MOFs nano-interface functionalization methods; (4) low utilization efficiency of MOFs nanomaterials. Therefore, it is highly required to construct more superior MOFs with engineered nano-bio interface to make MOFs more diverse, stable, efficient, and cost-effective for biomedical applications. Recently, Professor Li and his collaborators found a new method to control the self-assembly of MOFs nanomaterials. As is shown in Figure 1, in this method, the assembly of MOFs nanomaterials is mainly controlled by using DNA framework materials (tetrahedral DNA) (Figure 1). In particular, a variety of well-defined superstructures can be customized by adjusting the number of phosphate groups that coordinate with the catalytic Zr-MOFs to create polymer MOFs superstructures (Poly (MOFs)). It was also found that this superstructure could exhibit excellent catalytic activity and molecular recognition capabilities, and greatly amplified the output of the signal. The research team further constructed a colorimetric biosensor with excellent analytical performance based on the DNA framework-controlled Poly(MOFs), which could realize target analysis at the molecular level as well as at the cellular level, and could be used for the diagnosis of HER2-positive breast cancer.
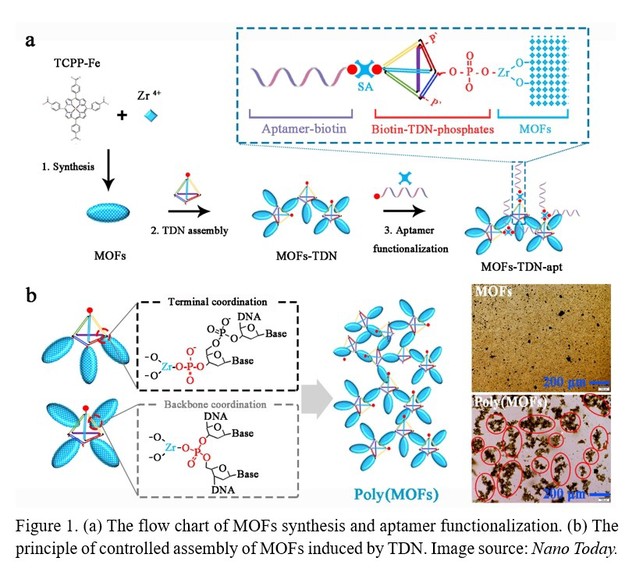
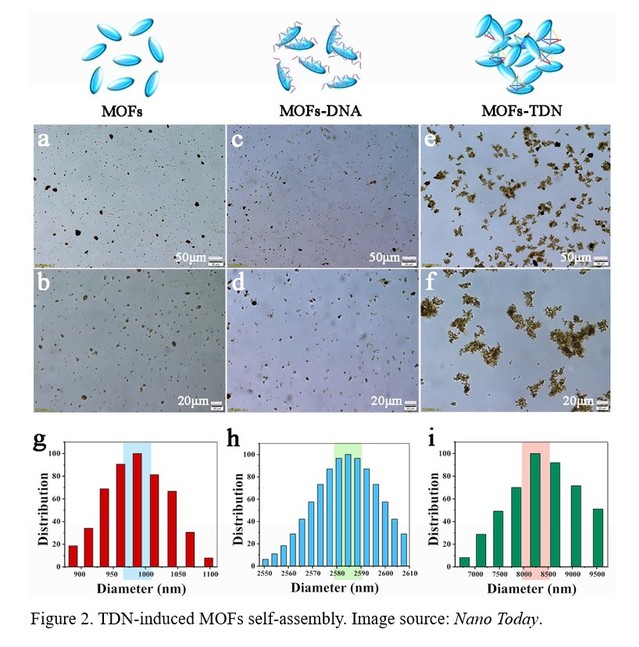
The researchers firstly explored the process of self-assembly of MOFs induced by tetrahedral DNA (TDN) and the key factors affecting the degree of self-assembly. The principle of TDN modification to MOFs is mainly through the coordination interaction between the phosphate group modified by TDN vertices and the zirconium ions on the surface of MOFs nanomaterials. It was found that single-stranded DNA modified MOFs could not induce the self-assembly of MOFs, while TDN could strongly induce the self-assembly of MOFs (Figure 2). The results showed that the more phosphate groups contained on the TDN vertex, the higher the degree of self-assembly of MOFs. Moreover, the concentration of TDN played a key role in the assembly of MOFs, and excess TDN or less TDN could not be conducive to the self-assembly of MOFs.
Subsequently, the researchers developed a visualization biosensor for the diagnosis of HER2-positive breast cancer based on the TDN-controlled self-assembly of MOFs (Figure 3). This biosensor mainly includes two modules: Magnetic beads (MBs) Trapping Module (MTM) and Signal Output Module (SOM) based on TDN-induced MOFs assembly. The MTM is obtained by linking a biotin-modified DNA aptamer to a streptavidin-modified MBs via the biotin-avidin system. As designed, the target (HER2) is firstly trapped and separated by multivalent DNA aptamer functionalized MBs (MBs-mApt) after rolling circle amplification (RCA). Then MOFs-TDN-apt recognizes and binds to HER2 captured by MBs. Finally, PCN-222 (Fe), a class of nanozyme-active MOFs, is used to catalyze the redox reaction of 3,3',5,5'-tetramethylbenzidine (TMB) into a blue oxidation state, thus highly sensitive measurement of the target can be achieved by a dual signal amplification procedure based on assembly of MOFs and RCA on the surface of MBs.
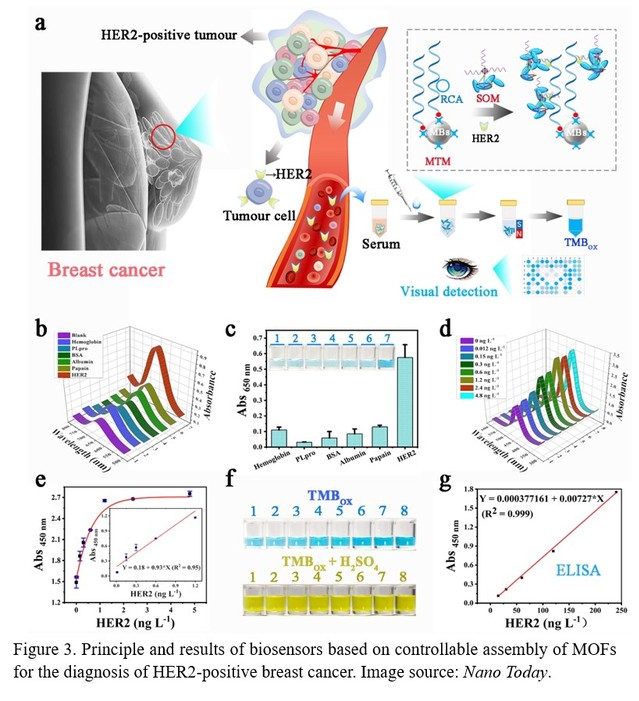
The researchers further verified the feasibility of the biosensor and screened two probes from three known DNA aptamers and a polyclonal antibody that can simultaneously and efficiently recognize the target. After optimizing the experimental parameters, the biosensor not only realizes stable and sensitive detection of the target protein at the molecular level, but also realizes the monitoring of the expression level of the target protein at the cellular level. Finally, the researchers validated the biosensor using serum samples from breast cancer patients, and found that it could effectively distinguish HER2-positive and HER2-negative breast cancer patients, indicating that it has great potential for clinical applications. This method is expected to provide a new method and optional adjunct for the early diagnosis of HER2-positive breast cancer and the monitoring of the efficacy of targeted therapy. Since the aptamers used in this work can be changed for other targets, while the assembly of DNA framework controlled Poly(MOFs) is the same, this work can be further extended for the assay of the biomarkers for other kinds of breast cancer, and even other diseases.
The above research results were published on Nano Today with the title of “DNA framework-controlled Poly (MOFs) as a visual platform for diagnosis of HER2-positive breast cancer” (https://doi.org/10.1016/j.nantod.2023.102143). Prof. Genxi Li, Prof. Yongmei Yin and Prof. Jing Zhao are the co-corresponding authors of the article, and Qizhi Liang, a doctoral student from the School of Life Sciences of Nanjing University, is the first author of the paper. This work was supported by the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, and the Graduate Research and Innovation Project of Jiangsu Province.
